Background: Pyruvate kinase (PK) deficiency is a rare, inherited hemolytic anemia caused by autosomal recessive mutations in the PKLR gene, whereby a glycolytic defect causes a reduction in adenosine triphosphate generation. Current treatment options are supportive and include splenectomy, blood transfusions, and iron chelation therapy. To better understand the natural history, treatment patterns, and burden of disease, the observational PK Deficiency Natural History Study (NHS) (NCT02053480) enrolled 254 adult and pediatric patients with PK deficiency at 30 sites in 6 countries between 2014 and 2017 and followed patients for 2 years. The Peak Registry (NCT03481738) was developed to continue and expand on this research. This retrospective and prospective observational registry aims to enroll 500 adult and pediatric patients at ~ 60 sites in up to 20 countries over 7 years, with 2-9 years of follow-up.
Objective: This analysis aimed to characterize the baseline demographics and clinical characteristics of patients with PK deficiency enrolled in the Peak Registry as of 24March2020.
Methods: Demographic, diagnostic, medical history, laboratory, treatment, and other relevant data were collected from participating clinicians via electronic case report forms. To be eligible for inclusion in this analysis, patients were required to have genetically confirmed PK deficiency and available demographic information. All analyses reported here are descriptive and based on data as of the date of enrollment in the registry. Continuous variables are summarized by the number of non-missing observations, mean, standard deviation (SD), median, and range. Categorical variables are summarized as counts and percentages.
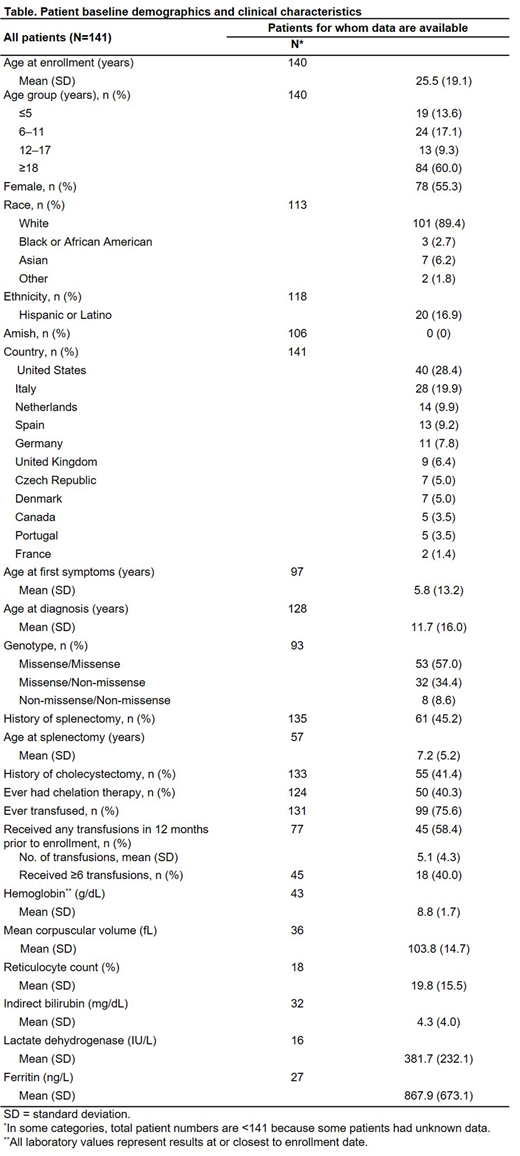
Results: A total of 141 patients met the inclusion criteria, across 11 countries in North America and Europe. A summary of baseline demographics and clinical characteristics is shown in the Table. Fifty patients (35.5%) had completed 2 years of follow-up in the NHS and then moved to the Peak Registry; the remainder were newly recruited to the Peak Registry. The mean age of study participants at enrollment was 25.5 years (SD 19.1); 78 patients (55.3%) were female. Mean reported age at first symptoms was 5.8 years (SD 13.2) and mean age at diagnosis was 11.7 years (SD 16.0). Fifty-seven percent of patients were classified as having missense/missense mutations, 34.4% as having missense/non-missense mutations, and 8.6% as having non-missense/non-missense mutations. The mean hemoglobin at enrollment was 8.8 g/dL (range: 5.8-12.9 g/dL). Mean reticulocyte count was 19.8% (range: 2.2-42.4%), mean lactate dehydrogenase was 382 IU/L (range: 135-849 IU/L), and mean indirect bilirubin was 4.3 mg/dL (range: 0.8-23.1 mg/dL). Among the 45.2% of patients who had been splenectomized, the mean age at splenectomy was 7.2 years. Chelation therapy had been previously prescribed to 40.3% of patients. Among the 27 patients for whom ferritin data were available, the mean was 867.9 ng/L (range: 78.1-2499.0 ng/L), and 18 patients (66.7%) had a level > 500 ng/L. Ninety-nine patients (70.2%) had received at least one transfusion in their lifetime. Among the 45 patients who were known to have received at ≥ 1 transfusion in the 12 months prior to enrollment, the mean number of transfusions during that period was 5 (SD 4.3), with 18 of those patients (40.0%) having received ≥ 6 transfusions.
Conclusions: New data emerging from the Peak Registry will provide valuable insights into the patient characteristics, treatment patterns, and burden associated with PK deficiency. The population is demographically heterogenous and represents a broad geography. Patients have a wide range of hemoglobin levels, and iron overload is common. The substantial rates of splenectomy, cholecystectomy, transfusions, and chelation use are indicative of a high disease and treatment burden in patients with PK deficiency.
This abstract is presented on behalf of the Peak Registry Steering Committee and Peak Registry Investigators.
Grace:Novartis: Research Funding; Pfizer: Research Funding; Agios: Research Funding; Dova: Membership on an entity's Board of Directors or advisory committees. Boscoe:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Bowden:Agios Pharmaceuticals: Current Employment, Current equity holder in private company. Glader:Agios Pharmaceuticals, Inc.: Consultancy. Layton:Cerus: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. van Beers:Novartis: Research Funding; Pfizer: Research Funding; RR mechatronics: Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding. Yan:Agios Pharmaceuticals: Consultancy. Bianchi:Agios Pharmaceuticals: Other: Scientific Advisor.
Author notes
Asterisk with author names denotes non-ASH members.